By Professor Dr. Serge Jurasunas
ABSTRACT
Past decades of research have established that the P53 tumor suppressor provides a major barrier to neoplasic transformation and tumor progression. Its role is not only associated with controlling cell cycle progression and apoptosis involving other apoptotic genes but is implicated in several functions including regulation of glycolysis, promoting oxidative phosphorylation, and repressing overactive telomerase enzymes.
First cancer cells acquire selective advantages by retaining mutant forms of P53 proteins that confer new oncogenic functions that upregulate several genes that accelerate tumor progression, metastasis, and chemotherapy resistance. In this article, we also demonstrate the new role of WT P53 in controlling telomerase activity since in most cancers telomerase is overexpressed which allows cancer cells to acquire a stem-cell-like state (CSCs), where these cells can continually renew becoming almost immortal.
Furthermore, they are responsible for most cancer recurrence and chemo-resistance. Mutant P53 cannot repress the enzymatic activity of telomerase, thus emphasizing the critical role that WT P53 plays in cancer therapy. We have now established a new ratio between P53 and telomerase that offers a new dimension and perspective to understand and treat cancer. However, several natural compounds have demonstrated efficacy to reactivate P53, inhibit glycolysis, and telomerase activity.
INTRODUCTION
Have we explored all the possibilities, new theories, new ways, new treatments to win the battle against cancer, or at least, to have a much better result? Probably not! Today in 2020 cancer has become an epidemic, while in 2005 Andrew Von Eschenbach, director of the NCI announced there would be an end to deaths caused by cancer by 2015. Would you believe such a thing at that time? But some doctors did believe in this prediction. In one article published in the same year, I read the following: “We are closer than ever from achieving this goal!”
It was just only a dream since today Modern Oncology is still in a deadlock since chemotherapy and radiation have not brought significant improvement to the longevity of cancer patients in the past 30-35 years, where cancer metastasis is still responsible for 90% of cancer deaths. Besides, Oncologists have absolutely no way to detect the risk of cancer recurrence or containment of primary tumors from metastasizing. How many times have we seen patients under chemotherapy developing more metastasis?
A 2012 study published in Natural Medicine strongly suggested that chemotherapy is responsible for disease recurrence. According to Dr. Dominique Belpomme, Professor of Oncology at University V Paris, today chemotherapy offers its maximum efficacy where we cannot expect more future progress since it has reached its upper limit (1). Therefore, the need for new modalities and tools for cancer prevention, cancer recurrence, and treatment with less toxicity, remain the main goal in modern oncology.
There is an urgent need to change the paradigm of cancer by searching for new theories and methods to treat cancer with more efficacy, some of which are newly emerging. This includes the theories of cellular respiration and the Warburg effect as the prime cause of cancer (2) Cancer as a metabolic disease is now gaining more interest in the scientific community. More recently, research has shown that the metabolic profile observed in cancer cells includes mitochondrial dysfunction, increased consumption of glucose, increased glycolysis and increased secretion of lactate, while oncogenes and tumor suppressors have been discovered to have an important role in cancer.
CANCER CELL RESISTANCE
Chemotherapy may kill many cancer cells sensitive to apoptosis but during the treatment, a small population can acquire apoptosis resistance by the up-regulation of multiple pro-survival factors such as loss of P53 gene function, activated inhibitors of apoptosis (IPAs), just as survivin, for example, increases the activity of the Nuclear Factor Kappa-B inducing lung metastasis of human breast cancer in nude mice treated with Paclitaxel but inhibited with curcumin (3). A tumor represents a diverse collection of cancer cells and when cancer is detected the million (or billions) of cells that make up the tumor have become with different cancer cells.
Some are differentiated cancer cells that are sensitive to chemotherapy and apoptosis, while others emerge during the late stage of the tumor development, being facilitated by the loss of P53, they function as undifferentiated Cancer stem cells possessing the ability to self-renew, become resistant and can usually originate metastasis. This type of cancer cell harbors a mutated P53 that has gained additional oncogenic function which we explain next when addressing P53 gene function more in depth.
P53 Function
Briefly, the P53 gene is a transcriptional factor, a potential master regulator with a broad range of biological functions that include primarily blocking cell cycle progression to induce apoptosis, senescence, DNA metabolism, and angiogenesis (4-5). To activate the apoptosis mechanism P53 transcriptionally regulates other apoptotic genes such as Bax while simultaneously suppressing the BCL2 antagonist (6). A potent anti-apoptotic oncogene that when activated blocks Bax in promoting apoptosis. (7) Both reduced Bax expression and overexpressed BcL2 is implicated with poor response to chemotherapy and shorter survival of cancer patients (8).
Thus we may better understand the role of WT P53 in the apoptosis process since a low P53 gene activity or even loss of P53 have some negative effects by not being able to regulate BcL2 and Bax expression. For instance, Bax is lost in 1/3 of breast cancers and BcL2 is activated in 60% of breast cancers, which contribute to drug resistance and shorter survival (9) Both BcL2 and Bax have potential prognostic and predictive significance. Several studies have shown that high Bax expression is associated with improving survival in a number of cancers. Both Bax and BcL2 are evaluated in a ratio that determines the fate of cells or the level of self-destroyed cancer cells.
The Bax/BcL2 ratio may be used as a predictive value for chemotherapy response and offer a better evaluation of what results can be expected (10). I have been able to observe and follow the Bax/BcL2 ratio with the blood testing we usually do with cancer patients. Thus we can improve this ratio by using select natural compounds. Another strong inhibitor of apoptosis is survivin which is not expressed in cancer tissue but undetectable in cancer tissue. This makes it not only a cancer marker but a promising therapeutic target in chemotherapy because of the resistance of cancer cells.
Survivin expression is observed in the most majority of cancers including breast and prostate cancer (11 12). Survivin was found to inhibit three apoptotic enzymes: caspase 3, caspase 7, and caspase 9, thus protecting cells from death. Survivin may act simultaneously with the BcL2 proteins, although differently as I have frequently observed with my cancer patients. Now Survivin is transcriptionally repressed by wild type P53 but not by mutant P53 (13), which again may explain the crucial role of wild type P53 in apoptosis and against cancer cell resistance during chemotherapy.
P53 Mutation
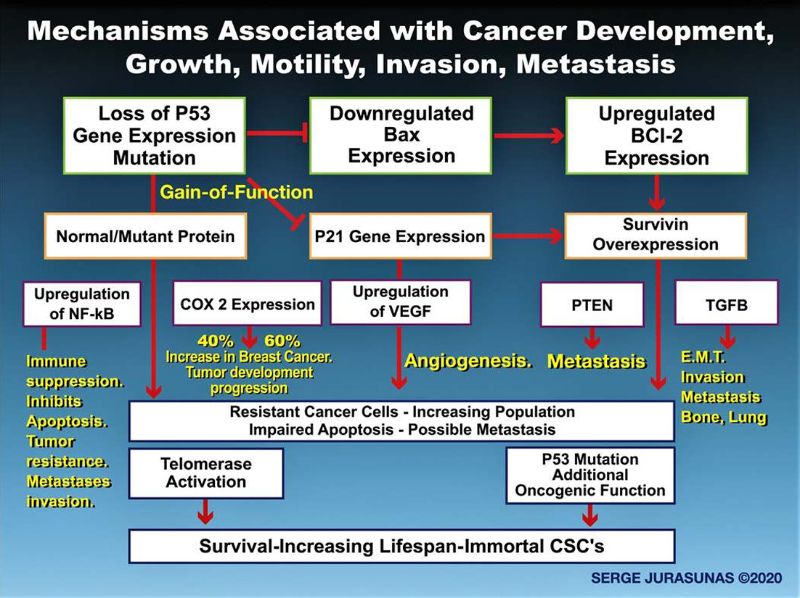
However, as bad news P53 itself is the most commonly mutated gene in the human body. It is harbored in more than half of all cancers and appears necessary to develop many forms of cancer. (14). In Oncology P53 mutation is a major obstacle to patient remission, causing cancer recurrence and decreased lifespan. P53 mutation not only blocks apoptosis but when mutated, P53 acquires additional functionality know as Gain-of-Function (GOF) (15), by change and up-regulation in gene expression that promotes tumor growth, angiogenesis, immune suppression, migration and metastasis invasion.
The P53 gene is part of a 6 network where cross-talking regulation occurs with a number of genes such as RAS, ID 4, E2F1, E-Cadherin, TGF-B, EGFR, C Myc, and even Nuclear Factor Kappa B. It is not surprising that loss of P53 or mutant P53 enhances NFK-B which in turn activates other mechanisms of tumor growth that when upregulated by mutant P53 contribute not only to resistance of cancer cells to chemotherapy but promotes tumor growth and metastasis invasion.
For instance, cancer cells can break the basal membrane and undergo Epithelial-Mesenchymal-Transition facilitated by up regulating TGF-B and acquiring migratory properties. Mutant P53 may induce EMT by up-regulation of TGF-B responsible for bone and lung metastasis in breast cancer (17) (See Figure 1).
When activated, WT P53 produces a protein that is degraded through the negative regulator MDM2, while mutant P53 protein is not degraded and accumulates. The more they accumulate, the more aggressive the cancer. For instance, I have often observed a high level of mutant P53 protein in all the aggressive ovarian cancer cases, for example, but managed to reverse it to a wild type protein, using natural compounds that I have selected after my extensive experience. Mutant protein eliminates the normal function of the WT protein by forming oligomeric complexes with WT protein through a dominant-negative effect, thus inactivating its function.
However, even with a WT P53, mutated protein can be produced and considered as a post-translational event during protein modification, while the mutated P53 gene is considered as a post-transcriptional mutation. P53 mutation (or P53 mutant protein) is associated with aggressive tumors increasing cancer cell resistance during chemotherapy but has been seen as a prognostic and predictive value for poor response, metastasis invasion, and increased recurrence during chemotherapy regimens. Some studies have found that in the presence of 7 mutated P53, in 14 out of 19 cases, the mutation was associated with a poor response to various chemotherapy or radiotherapy regimens in breast, colorectal, ovarium, stomach and soft tissue carcinomas.
65 out of 93 studies found that P53 is a statistically significant factor of poor prognosis in various cancers. Texas researchers have found that high levels of P53 mutated protein accumulation are associated with a significant increase in local recurrence rate in a study of 1,500 breast cancer patients treated with mastectomy and chemotherapy. There was about a 40% lifespan reduction in breast cancer patients at 5 years survival, harboring a P53 mutation, compared to patients with a WT P53 (19).
P53 Inhibits the Warburg Effect by Reducing Glycolysis and Enhancing Oxidative Phosphorylation
Besides being a tumor suppressor gene associated with apoptosis P53 has other important functions but less known or even ignored. These provide another approach to one of the main Hallmarks of cancer known as the Warburg Effect (20) by regulation of glycolysis and shutting down glycolysis transporters and other expressions of glycolysis enzyme promoting oxidative phosphorylation (oxphos) through transcriptional regulation of target genes. P53 can both inhibit glycolysis but when mutated or misfolded it stimulates glycolysis that cancer cells use to generate energy and contributes to cancer progression.
Thus, by P53 balancing the use of glycolysis and oxphos it provides a mechanism blocking tumorigenesis and the Warburg effect. Glycolysis is the pathway that tumors use as an alternative for generating energy via oxidative phosphorylation or respiration in 8 mitochondria to generate the so-called ATP molecules from sugar burned or oxidized in the presence of oxygen and broken down into C02 and H20.
Due to oxygen reduction in the mitochondria and blockage of the cellular respiratory chain from deficient respiratory enzyme and other Electron Chain transport damage (or mtDNA mutation (observed in a variety of human cancers), this may further contribute to respiratory malfunction in cancer cells. The German Biochemist Otto Warburg proposed in 1924 that cancer was caused by a defect of oxphos or respiration in the mitochondria forcing cells to switch to the old primitive fashion when oxygen cannot be used by the mitochondria to generate cellular ATP energy, by switching to the glycolysis channel (21.22.23). Otto Warburg who was the director of the famous Max Planck Institute for Cell Physiology in Berlin won his first Nobel Prize for physiology and medicine in 1931 for the oxygen transferring enzyme of cell respiration and his second Novel Prize in 1944 for his discovery of hydrogen transferring enzymes.
This latter process does not require oxygen because it arose early in the primordial evolution of prokaryotic cells back when the earth’s atmosphere had very little oxygen. Warburg analyzed the ratio of Oxphos to glycolysis in different cancer cell tissues and found that glycolysis under anaerobic fermentation was particularly high in aggressive tumors when compared with benign tumors and normal tissues. These observations led Warburg to propose a deficiency in oxphos and elevated glycolysis as the primary cause of cancer.
Today the Warburg effect that now is enjoying a resurrection and has started to be taken very seriously by several researchers and even doctors and oncologists.
Phosphorylation and Aerobic Respiration are Found in Mitochondria-You can Easily Understand the Primordial Role of Mitochondria in the Cause of Cancer.
The glycolytic pathway is found in the cytoplasm of each cell that does not require oxygen
However, glycolysis pathways produce ATP less efficiently than aerobic respiration resulting only in the production of 2 molecules of ATP per molecule of glucose while and 36 molecules of ATP are produced in oxphos, while this is not enough energy for cancer cells yet cancer cells possess a 20 to 30 fold increased rate of glucose cellular uptake and a more than 30 fold higher glycolytic rate when compared to normal cells. Cancer cells produce ATP from glycolysis a hundred times faster than normal cells (24) in order to support three basic needs of these cells: maintenance of energy status, increased biosynthesis of macromolecules such as proteins, as well as maintenance of the cellular redox status which permits survival and growth.
Several studies have already shown today that WT P53 plays a crucial role in slowing down or inhibiting glycolysis and at the same time promote oxphos and cellular respiration through transcriptional regulation of target genes. While mutant protein or even misfolded protein which has lost its anti-tumor capabilities triggers glycolysis to favor cancer progression (25). Since P53 is mutated in many tumors and thus can influence aspects of both glycolysis and oxphos, thus being significantly important in contributing to the Warburg effect.
How Cancer Cells Use Glucose
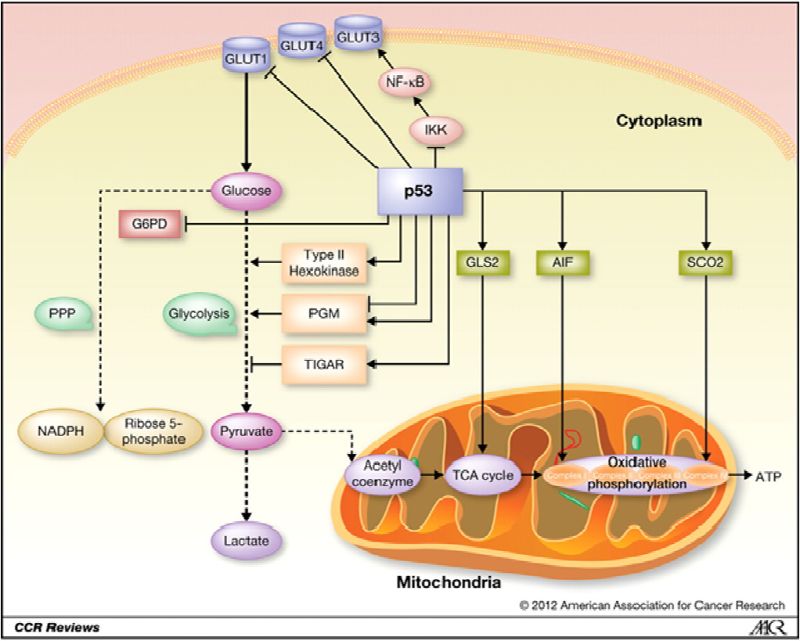
Cancer cells use glucose to generate energy whose entrance into the cells from the increase of the glucose receptors on the surface is differentially mediated by glucose transporters and then converted in pyruvate which is then further converted in lactate. Cells with high rates of glycolysis produce more lactate and exhibit decreased mitochondrial respiration compared to cells with WT P53 indicating that WT P53 suppresses aerobic glycolysis. The family of transporters that mediate the transport of glucose is comprised of four major transporters including GluT1, GluT2, GluT3, and GluT4. Each of the GluT transport proteins possesses different affinities for glucose and other hexoses such as fructose. GluT1, GluT2, GluT3, GluT4 have a high affinity for glucose allowing transport of glucose at a high rate under normal physiological process (26) but also by the tumor cell.
Tumor cells badly need glucose thus increasing its cellular intake associated with increased and deregulated GLUT transport expression. In fact, glucose uptake mediated by GLUT1 appears to be critical in the early stages of breast cancer development, affecting cell transformation and tumor formation (29). But other glucose transporters such as GLUT2, GLUT3, and GLUT4 are also expressed in breast cancer and other cancers (30, 31), correlating with a poor prognosis (32). GLUT1 and GLUT3 are associated with cancer cell resistance to radiotherapy or chemotherapy (33).
Another example of P53 directly represses the transcriptional activity of GLUT3 gene expression indirectly by preventing activation of the IKK-NF-KB pathway As already described WT P53 can reduce intracellular uptake of glucose by downregulating the glucose transporters by directly repressing gene coding resistance for the glucose transporter (34.35), while mutated P53 gene expression stimulates glycolysis which is an additional oncogenic function of the mutated gene.
However, WT P53 can use other channels to regulate glycolysis by promoting the expression of the Tigar gene (TP53-induced glycolysis and apoptosis regulator) and by down-regulation of PGM (phosphoglycerate mutase). Tigar is gene-regulated as part of the P53 tumor suppressor pathway (36) which encodes a protein similar to glycolytic enzymes. This recently discovered enzyme primarily functions as a regulator of glucose in human cells. The protein functions by blocking glycolysis and also protects cells from DNA damaging Reactive Oxygen Species, further providing some protection from DNA-damage-induced apoptosis. PGM is a derived polypeptide that can inhibit glycolytic flux. WT P53 downregulates PGM but mutated P53 to the contrary enhances PGM activity, thus increasing glycolysis flux. Therefore, the downregulation of PGM expression and activity by WT P53 can inhibit the glycolysis pathway (37).
P53 Promotes Oxidative Phosphorylation
One of the important but probably less known functions of P53 is the regulation of the metabolic versatility of cells by favoring mitochondrial respiration over glycolysis, oxidative phosphorylation (oxphos), and reversal of the Warburg effect. In mitochondria, the key components involved in oxphos and MT respiration are cytochrome C oxidase (SCO2) synthesis (critical for regulating a major site of oxygen utilization by the cell) and cytochrome C oxidase (COX), which can be directly trans activated by WT P53. SCO2 is required for the assembly of the MT DNA encoded cytochrome C oxidase (COX II) sub-unit into the COX complex in the mitochondria Electron Transport Chain, serving as the site of mitochondrial oxphos in mammalian cells. P53 supports a switch from aerobic respiration to glycolysis through the disruption of COX2 function which decreases cellular dependence on oxygen. Therefore, SCO2 disruption with mutant P53 in human cancer cells aggravates the metabolic switch into glycolysis. Activation of WT P53 could increase SCO2 expression and thereby stimulate MT respiration and ATP production (38,39).
Furthermore, other studies have shown that WT P53 can directly upregulate the COX I gene encoding sub-unit of the COX complex found in colon cancer cells which may contribute to the maintenance of MT cytochrome C oxidase and complex IV in the Electron Transport Chain. Finally, P53 can also transcriptionally activate the apoptosis-inducing factor, (AIF) to maintain the integrity of complex I in the MT Electron Transport Chain, through maintaining the MT respiratory chain. Thus WT P53 plays an important role in enhancing MT oxphos in cancer cells, reactivating oxphos, and shutting down glycolysis, all being considered as a potential cancer therapy.
Telomerase Activity and P53 Tumor Suppressor Gene in Cancer
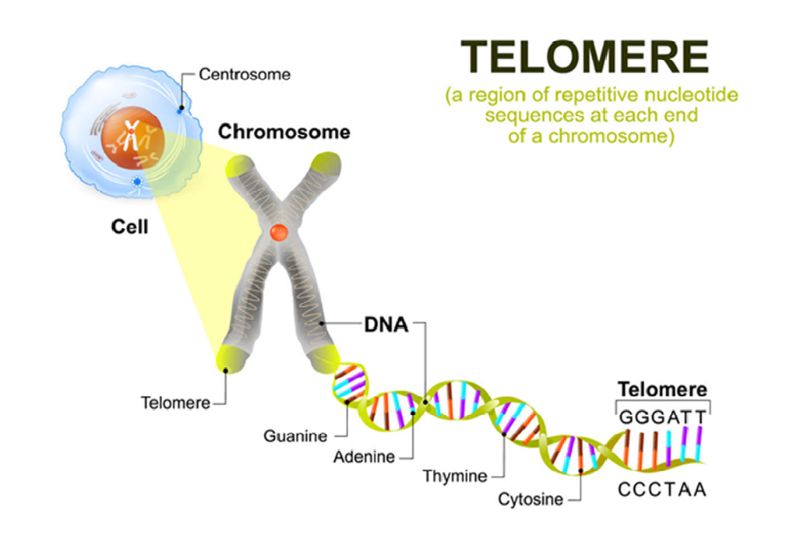
A growing number of studies are now showing the implication of Telomerase overexpression in the malignant transformation and progression of the human tumor (40). Telomerase is a large ribonucleoprotein complex in nature, a reverse transcriptase enzyme that carries its RNA molecules which are used as a template when it elongates telomeres. They consist minimally and essentially of the protein catalytic subunit (hTERT) coded for human Telomerase reverse transcriptase and telomerase associated protein (TEP1). Its role is to maintain telomere integrity in the sequences of the ends of eukaryotic chromosomes and to prevent the ends from undergoing a DNA damage response, playing a critical role in chromosome replication.
The activity of telomerase is to regulate dividing cells and to maintain the telomere ends against erosion at their normal length. As we age and under some circumstances, for instance, have excess oxidative stress or radiation, telomeres gradually shorten. This shortening leads to cell aging, cell death or senescence; however, some somatic cells do not have sufficiently high levels of telomerase to maintain the length of their telomere for an indefinite number of divisions, consequently, the telomeres gradually shorten as cells age. This is why telomere activity has been widely studied as an aging factor (41).
However, while normal somatic cells show no telomerase activity, cancer cells express abundantly high telomerase and thus may be considered as a relevant factor distinguishing cancer cells from normal cells. It’s as if cancer cells use telomerase to divide indefinitely. Telomerase up-regulation/reactivation has been observed in 85 % of all human tumors. Such tumors may become immortal and play a crucial role during human tumor parthenogenesis (42). Besides being found in primary tumors, telomerase activity is also detected in circulating tumor cells for instance in breast (43), ovarian (44), and prostate cancers (45).
Many cancer cells are considered immortal because telomerase activity allows them to divide virtually forever because they possess the ability to continually regenerate their telomeres (46). These like cancer cells become resistant and under several circumstances, they acquire some stem cell characteristics known as a cancer stem cell-like state (CSCs). Since to survive they need to adapt to an ever-changing environment where they undergo a genetic change from metabolic change, oncogene activation, epigenetic change, tumor suppressor gene inactivation, shift to aerobic glycolysis, etc. They develop a very strong DNA repair mechanism, more rapidly than normal cells. While chemotherapy can damage them, they can repair quickly.
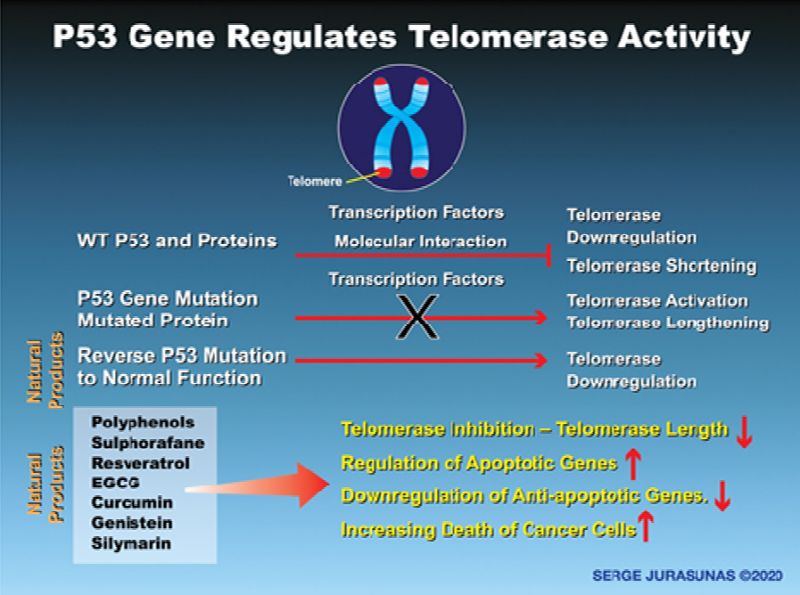
Now the P53 tumor suppressor gene, independently of inducing cell cycle arrest and apoptosis, seems to also play a crucial role in protecting telomeres from DNA damage and regulating telomerase activity through a crosstalk node between these 2 important mechanisms (47). According to a recent discovery led by Paul Lieberman of the Wistar Institute, this is particularly a new function of P53 that had never been described. Both telomeres and P53 play an important role in the maintenance of genome integrity and the tumor suppressor, and easily could be regarded as Guardian of the genome (48). The local binding of P53 to the region close to the amino terminus of telomerase associated protein 1 (hTEP1) is one response to DNA damage protecting the telomere.
But also wild type P53 represses the enzymatic activity of telomerase through downregulation of its protein catalytic subunit, human telomerase reverse transcriptase (hTERT) together with the interaction of the SP1 protein transcription factor. Wild type P53 protein level prevents telomerase from becoming overactive and proliferating indefinitely, while P53 mutation and accumulation of mutant protein correlates with telomerase over activity in many cancers, ovarian cancer (48) and breast cancers (49-50) and non-small cell lung cancer (NSCLC) (51). I have also personally observed this during my career with other cancers in our patients such as bladder cancer, sarcoma, and prostate cancer.
With the activation of telomerase, some types of cells and their offspring possess the ability to continually regenerate their telomere, divide continuously increasing their lifespan and immortality. On the contrary, Telomerase shortening provides a barrier to cancer progression where the majority of cancer cells depend on telomerase activation to gain proliferative immortality. If the P53 gene is more activated than telomerase cancer cells in all probability are not yet immortal, they have a limited lifespan and can be destroyed. If telomerase is more highly activated then p53 gene cancer cells are out of control and become immortal. This is why P53 gene expression leads to more effective control over the telomerase activity and prevents cancer cells from becoming immortal cells.
Telomerase Upregulation/Reactivation is observed in 85% of all Cancers Up to 90% in Breast Cancer Suggesting a Crucial Role during Human Tumor Pathogenesis.
The P53 Tumor Suppressor Gene is Mutated in 50% of All Cancers
Both P53 mutation and overexpressed Telomerase are considered an important event in earlier cancer development and progression of the disease with metastasis invasion. Overexpression of WT P53 was shown to down-regulate the telomerase enzymatic activity.
The role of the P53 tumor suppressor gene is to kill cancer cells via apoptosis and prevent them from continuously dividing, thus preventing cancer cells from reaching a more aggressive stem-like state.
Mutant P53 and overactive telomerase allow cancer cells within a tumor to turn back time by acquiring a stem-cell-like state (CSCs) by developing survival factors
This type of aggressive cell usually emerges during the later stage of tumor development facilitated by the loss of P53. They possess the ability to self-renew, differentiate by forming resistant phenotypes and originating metastasis activity, chemo-resistance (52.53), failure of treatment, and tumor relapse. These CSCs display high levels of telomerase activity possessing the ability to continually regenerate their telomeres (54). The WT P53 tumor suppressor gene indeed plays a more crucial role than we may have initially learned, such as inducing apoptosis. When activated with the production of normal protein it prevents established cancer cells from moving toward a more aggressive stem-like state, especially by regulating telomerase activity.
Now first if the tumor suppressor gene is malfunctioning due to a lack of protein production or mutated protein, telomerase can become overactive. This means that along with the transformation of cells with mutated P53 into cancer cells, there is a high probability they also may be able to form a tumor. Fortunately, we can evaluate the presence of any cancer cells capable of forming a tumor in the case of a non-cancer patient, meaning we start at a preventive level. Now, what is important regarding cancer patients under treatment or even best before starting treatment is to evaluate the activity of these two genes with a ratio of telomerase/P53.
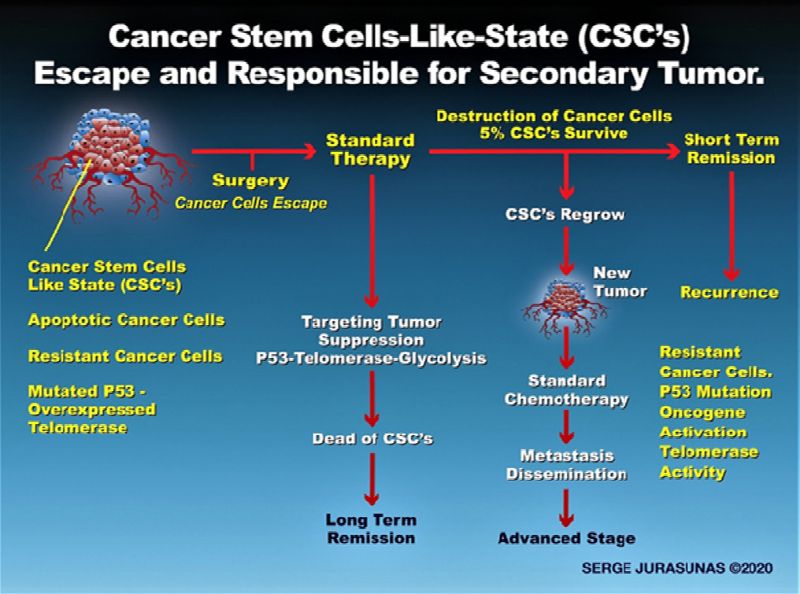
We have to know if the P53 gene is more activated than the telomerase or vice versa to determine the presence of non-resistant CSCs or in a case of cancer remission if there remains a presence of cancer cells somewhere in the body with a high risk of recurrence.
On several occasions, I have seen as a result of testing cancer patients in remission a bad 53/Telomerase ratio, including the presence of mutated P53 protein, where we already know by experience that cancer recurrence is still high in such breast cancers. We have not come across any other techniques that permit clinicians to evaluate the risk of a recurrence. P53 mutation by itself together with BcL2 overexpression represents a sign of high-risk for cancer recurrence as I have often observed in several breast cancer patients. New studies have shown that TERT overexpression upregulated the expression of BcL2 and downregulated Bax activity reducing the activation of some caspases proteins such as caspase 9 (55.56).
This explains why sometimes P53, even if it is highly expressed, BcL2 is also expressed even if is normally activated, but BcL2 can be overexpressed because of overexpression of TERT in the telomerase. There is a feedback mechanism between the two that we will develop further in my blog. Thus a bad P53/Telomerase ratio suggests the presence of CSCs with resistance to apoptosis and chemotherapy. Remember that overactive telomerase provides immortality to cancer cells if not regulated by WT P53 and the production of normal protein.
Telomerase activity may be seen as a new marker for cancer. In fact, the levels of telomerase activity (independent of the P53 gene) in the early and late stages of cancer might be used to determine the diagnosis of various human cancers and as a biomarker for detection.
RATIO: P53/TELOMERASE ACTIVITY – A NEW WAY TO DIAGNOSE CANCER
For nearly 15 years I’ve been using, based on my clinical experience, observation, and from studying a variety of selected natural compounds that exhibit anti-tumor activity targeting several mechanisms used by the tumor for growth and expansion. One of the most important strategies is to reactivate (restore) mutated P53 to a normal function, such as normal P53 protein production level, together with downregulation of telomerase activity and decreasing glycolysis. For this purpose, we can just perform a blood test with molecular markers. We include P53 gene expression, P53 protein level, BcL2, Bax, Survivin, P21 gene expression and proteins, VEGF, MMPs, TNFa, and telomerase activity.
I do believe that targeting P53/Telomerase activity remains a major challenge in Oncology. Theoretically, science has been researching a synthesis compound or drugs that can inhibit telomerase. All the while, we have on hand a range of natural compounds that have already demonstrated efficacy to inhibit telomerase activity with no side effects, as I am going to explain.
In order to offer such services, we work with a Laboratory specializing in Biological Assays directed by Dr. Olga Galkina Taylor PhD., a highly reputed Russian scientist who opened a new door in my professional life. We conjointly wrote an article in the Townsend Letter in 2010. The article explains how to reverse P53 mutation with my own therapy protocol (57), but probably it came out too soon to be well understood at that time. We take a venous blood sample from the patient before treatment, to determine an exact genetic condition between the pro-tumor activity and anti tumor activity; for instance the ratio Bax/BcL2. Since P53 gene expression regulates telomerase activity, we have concluded that we may also define a P53/Telomerase ratio, then use it as a diagnosis, prognosis, and prescribe the appropriate treatment according to the result.
If WT P53 is overexpressed and it downregulates telomerase enzyme activity then the cancer tumor is much less resistant and more sensitized to chemotherapy. We started doing P53/Telomerase activity only a couple of years ago but so far we have collected many interesting cases. We perform a second test (when possible) to verify the effect of our treatment on the expression of the various genes included, and then observe the results. Some patients even after remission continue with regular testing even after several years for purposes of prevention and monitoring recurrence. Often the results have shown a condition of high-risk recurrence where we immediately modify the patient’s regimen with appropriate treatment. This is the best way to handle cancer disease and especially prevent any recurrence. Of course, I performed very extensive research and study into the many roles of telomerase in both aging and cancer, especially on the new link between P53 and telomerase and its application in cancer.
I was then highly motivated to research which natural compounds had the best efficacy to inhibit telomerase activity while applying the knowledge I gained years ago about restoring mutant P53 to the WT P53 tumor suppressor gene, using selected natural compounds.
In the presence of mutant P53 and highly activated telomerase, we definitively need to first reactivate the gene or the production of normal protein over mutated protein. This is more complicated than only increasing the P53 gene activity. Many natural compounds can stimulate P53 gene activity to increase apoptosis and to regulate telomerase, but not all natural compounds can reverse a P53 mutation but I have been able to reverse it. Once again please refer to the article previously mentioned, describing in detail the list of the natural agents that have demonstrated efficacy to target both apoptosis, telomerase (58), or interfere with glucose transport (59). However, this topic is too involved for this article. We will just provide the name of the most important agents with some references, but will later offer more details and references, interesting figures for the reader in my blog along with complete molecular markers clinic case reports with a full explanation.
Again this requires considerable space but it’s very important for doctors to understand how to more effectively handle a cancer case and see how the applied therapy is functioning and the genetic status is improving. Today we have enough scientific proof of the multiple targeted cancer therapies utilizing curcumin and genistein, which for instance, induces apoptosis, activates the immune cells, inhibits NF-KB, HER-2/Neu, EGFR, downregulates BcL2, and survivin upregulates Bax, decreases COX2 activity, activates caspases and stimulates the immune system. (60). But what is important is the fact that both curcumin and genistein have strong properties to inhibit telomerase activity by decreasing the level of TERT (61.62.63). Both curcumin and genistein may kill CSCs (64.65) more effectively than chemotherapy.
This explains why for many years I have included liposomal curcumin and genistein in my cancer protocol together with Rice Bran Arabinoxylan Compound (RBAC), a strong Biological Response Modifier that has special properties to activate NK cells. The reader can refer to my last article in the Townsend Letter 2019 (66). Besides being known as a strong immunomodulator some new studies have shown that RBAC improves the ratio BcL2|Bax with radiotherapy (67). If you use these 3 compounds together in your cancer protocol and maybe include an anti-antiangiogenic therapy for instance, like C-Statin, made from a naturally occurring plant extract (bindweed) that contains a proteoglycan molecule with strong angiogenic properties.
You may observe how the tumor can decrease in size and/or eliminate metastasis (See my article How to Approach Cancer Patients, diagnostic and treatment with figures of cases-August/Sept 2018. 68-73). But of course, other natural compounds have shown efficacy to repress telomerase activity besides targeting other cancer mechanisms such as apoptosis, including resveratrol (68) the green tea polyphenol, epigallocatechin-3-gallate (69), Allicin (70), sulphoraphane (71), silymarin (72), Quercetin (73) and Genistein (74).
Example with a Ratio of P53 Gene and Telomerase Activity.
Here we present 3 patient case examples, but incomplete concerning the other apoptotic and anti-apoptotic genes again due to space limitations but will show more detailed complete reports along with more cases, in my blog.
Clinical case No.1: Female, 66 years old a medical doctor living and practicing in the US with breast cancer remission after surgery and chemotherapy. A very emotive person, under high-stress condition, anxiety. Have started with a better dietary style, does meditation and relaxation. The test was done several months after she started my suggested treatment and diet.
The last test was taken in October 2019
P53 gene expression: 6116 units/ml of plasma
P53 normal protein level: 1.520 units/ml of plasma.
Reference range: 0.1-1.00 unit
P53 mutated protein level: 28.5 units/ml of plasma.
Reference: (N.D. Trace)
P53 misfolded protein: 500 units/ml of plasma.
Reference: (N.D. Trace)
Telomerase activity: 6.816 units. Reference (N.D. Trace)
The ratio between P53 gene expression and telomerase gene
Expression: 0.9…(Ref range. 1.)
The P53 gene expression is highly activated together with a very high level of normal P53 protein probably due to the applied therapy and many damaged precancerous cells were destroyed as indicated by the test. However, some cancerous cells started to produce mutated protein and a high level of misfolded protein that can trigger glycolysis. It also may compromise the destruction of some populations of cancer cells through apoptosis altogether.
The P53/Telomerase ratio is a little bit lower, but not enough at the moment to create very resistant cancer cells. The apoptosis is process is highly activated. However with time, if not improved together with the elimination of mutated and misfolded P53 protein, she may develop resistant cancer cells and increase the risk of recurrence. However, through April 2020 the patient remains in remission. (See more details in my blog: NaturopathicOncology.blogspot.com).
Clinical case No.2: Female, a 38-year-old pharmacist living on the Island of Madeira, diagnosed with lymphoma but has refused chemotherapy. She went to Germany to receive a special dendritic cell vaccine and ozone therapy treatments.
March 2019: Test Results:
P53 gene expression: 380 units/ml of plasma
P53 normal protein level: N.D.
P53 misfolded protein level: N.D.
P53 mutated protein: 13.2 units/ml of plasma
Telomerase activity: 3.108 units/ml of plasma
The ratio between P53 gene expression and telomerase activity: (N.A.)
Here we cannot determine a ratio, with the results being too low together with the high expression of BcL2 and survivin, with a very low BcL2/Bax ratio. It shows the presence of cancer cells that are very resistant to destruction (see more details in the blog). P53 gene expression was too low, producing no normal protein level but if mutated can trigger glycolysis and inflammation. There is an increasing population of cancer cells with active telomerase and only a small fraction had been destroyed. Because of the high activity of the telomerase compared to the P53 gene activity many cancer cells can turn into CSCs. Here only a small fraction of pre-cancerous/cancerous cells were destroyed through the genes, however many cancer cells may be destroyed via the immune cells, especially by NK cells which have increased their activity after the treatment in Germany as observed in the report from the clinic. So the immune system and especially the NK cells are an alternative to destroy cancer cells when tumor suppressor genes alone are not efficient.
Clinical case No. 3:
Male, 16 years old diagnosed with an Ewing sarcoma. This is a very difficult case that I treated for 7 years but with very good results so far. Surgery could not remove the total tumor localized on the spine. The boy was subjected to several surgeries, chemotherapy, and radiotherapy yet improved considerably with our treatment. Recently we performed molecular markers testing including telomerase, in order to have a better prognostic and possibly prevent any recurrent cancer activity. As of the last Scan, the remaining tumor tissue was inactivated.
P53 gene expression: 5.915 units/ml of plasma
P53 normal protein level: N.D.
P53 misfolded protein level: N.D.
P53 mutated protein: 20 units/ml of plasma
Telomerase activity: 4,388 units/ml of plasma
The ratio between the P53 gene and telomerase activity: 1.3
The patient continued taking my treatment for the past 7 years which is a success. The high P53 gene activity (before: 1.134) is probably from the applied treatment but no normal P53 protein was produced but only mutated protein. However many cancer cells were destroyed through the highly activated Bax and P21 gene expression. (See my blog) The ratio P53/Telomerase is good 1.3; P53 gene expression maintains a lower telomerase activity and an inactivated tumor. However, the result of the test showed the presence of cancer cells somewhere in the body but the patient continues with check-ups to know but the risk of tumor reactivation still exists (We still closely follow the case).
More information on cancer can be seen from my last lecture in Europe on Slideshare: www.slideshare.net/sheldonstein.
“How to Understand and Treat Cancer with Modern Methods”
28th Feb. 2018 Zagreb. Croatia
The reader may receive an in-depth understanding by viewing my 2019 Medicine Week (Baden-Baden) Presentation on Slideshare:
“A New Modern Way to Approach Cancer”
https://www.slideshare.net/SheldonStein/prof-serge-jurasunas upcoming-presentation-oct-30-2019-baden-baden
References:
- Prof. Dominique Belpomme. Guerir du cancer ous! en proteger. Edition Fayard. Paris 2005. p.166-167
- Warburg O. On the origin of cancer cells. Science 1956.123. (3191). 309-314.
- Bharat B, Aggarwal, Shishir Shishodia, Yasunari Takada. Sanjeev E. Bueso-Ramos and Janet E Price. Curcumin suppresses the paclitaxel-induced Nuclear Factor K-B in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Cancer Therapy. Preclinical- Published October 2005.
- Aggarwal ML. Taylor WR, Chernow MV et al. The P53 network. J. Biolo Chem. 1998. 273-1-4.
- Vogt Sionow R, Haupt Y. The cellular response to P53. The decision between life and death. Oncogene. 1999.18. 6145-6157
- Miyashita T, Krajewiki S, Krajewska M et al. Tumor suppressor P53 is a regulator of BcL2 and Bax gene expression in vitro and in vivo. Oncogene. 1994-9-1799-805.
- Hockenberry D.M et al. BcL2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature (London) 199. 548.334-336.
- Krajewski C, Blomquist K, Fransilla M, Krajewski VM, Wasenius E, Niskamens, Nordling, J.C Reed. Reduced expression of pro-apoptotic Bax is associated with a poor response rate to combination chemotherapy and shorter survival in women metastatic breast adenocarcinoma. Cancer Res 55. 1995. 4471 4478.
- Mohammed Hassan Naseri, Majid Mahdavi, Shima Hallai, Shima Hallai Neishabouri. Up-regulation of Bax and up regulation of BcL2 during 3-nc mediated apoptosis in human cancer cells. Cancer Cell Int. 28 May 2015.
- Scopa Chriscula, Vogronas, Constantine, Kardinaki, Dimitris Kourelis, Athanasias C. BcL2/Bax ratio as a predictive marker for therapeutic response to radiotherapy in a patient with colorectal cancer. Immunocytochemistry. Molecular Morphology. Dec 2001. Vol 9. issue 4.328-334.
- Kum Kum Jha et al. Survivin expression and targeting in breast cancer. Surg Oncol June 2012.
- Shakrokl F. et al. Survivin expression is associate with features of biologically aggressive prostate carcinoma. Cancer. February 15.2004-100 (4).
- Mirza A, et al. Human survivin is negatively regulated by wild type P53 and participates in P53 dependent apoptotic pathways. Oncogene. 2002. 21. 2631-22.
- Noa Rivlin, Ran Brosh and Varda Rotter, Mutation in the P53 Tumor suppressor gene. Genes Cancer. 2011. April 2 (4) 466-474
- Oren M, Rotter V. Mutant P53 Gain-of-function in cancer. Cold Spring Harb. Perspect Biol. 2010-2 a001107.
- Wikstroom P, et al. Transforming Growth Factor beta is associated with angiogenesis in metastasis and poor clinical outcome in prostate cancer. The prostate 15 sept.1996.
- Antonella Chechi, David L. Waning, and Khalid S. Mohammed. The role of TGFB in breast cancer bone metastasis. Advances in Bioscience and biotechnology. 2013. Oct 1-4 (10c) 15.30. Published online 2013 Oct 13.
- Aas T, Berresen AL Geishler S, Smith Sorensen B, Johansen H, Varlang, Aksklen L, Lenning P. Specific mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nature Medicine 1996. 2811-2814.
- Oliver et al. TP53 mutations are associated with shorter survival in breast cancer independently of stage, grade, and hormone receptors status. Clin. Cancer Res 2006.
- Warburg O. On respiratory impairment in cancer cells. Science 1956. 124. (3215) 269-70.
- Warburg O. Uber den Stoffwechsel der carcinomzell. Die Naturwissenschaften 1924. 12. 1131.1139.
- Warburg O. The Prime Cause and Prevention of cancer, (lecture at the meeting of the Nobel-Laureates on June 30.1966 Germany. Published by Konrad Triltsch, Wurzburg, Germany 1969. English edition by Dean Burk. National Cancer Institute. USA.
- Warburg O, Versuche an Ubeeeerlebendem carcinom-Gewebe ( Methodend) Biochem Zeitschr 142. 317-333 .1923.
- Demetrius LA, Coy JF, Tuszynski JA (2010) cancer proliferation and therapy. The Warburg effect and quantum metabolism. Theoretical Biology. Medical Modeling. 7.2.
- Ambroise Gorbatchev, Amanda Ouchida, Andre Lima Queiroz. The effect of mutant P53 proteins on glycolysis and mitochondrial metabolism. Molecular and Cellular Biology .17 Oct 2017. 37 (24)
- Archana M. Navale and Archana N Paranjape. Glucose transporters. Physiological and pathological roles. Biophys Rev 2016 March. 8 (1) 5-9.
- Matilda Eriksson et al. Effect of mutant P53 proteins in glycolysis and mitochondrial
- Szalbewski L. Expression of glucose transporters in cancer. Biochim. Biophys- Acta. 2013. 1835. 164-169.
- Krzeslk A, Wojcik. Krowiranda K, Forma E, Jozwak P, Romanowicz H, Bienkiewiez A, Brys M. Expression of GLUT4 and GLUT3 glucose transporters in endometrial and breast cancers, Pathol. Oncol. Res 2012. 18. 721-728
- Macheda ML. Rogers S. Best J.D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell Physiol. 2005. 202-654-662.
- Medina RA. Owen GI. Glucose transporters. Expression, regulation, and cancer. Biol Res. 2002. 33-9-26.
- Fernanda Rocka Alaya et al. GLUT1 and GLUT3 as potential prognostic markers for oral squamous cell carcinoma. Molecules 15 (4) 2374-87. April 2010.
- Kuand R, Jahangiri A, Mascharak S, Nguyen A, Chandra A, Flamgain PM, Yagnik G. Wagneeeer JR, De Lay M, Carreira D et al. GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance. JCI insight 2017. 2 e88815.
- Scwartzwnberg-Bar-Yoseph F, Armoni M, Karmeli E. The tumor suppressor P53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 2004.64 (7) 2627-33.
- Cen Zhang, Juan Liu, Zhaohui Feng. Tumor associate mutant P53 drives the Warburg effect. Nature Communication 4. Article number 2935. (2013)
- Bensaad K, Tsuruta A, Selak MA, et al. Tigar, a P53-inducible regulator of glycolysis and apoptosis. Cell 2006. 126 (1) 107.20.
- Kondoh Hiroshi, Leleonart ME. Gil J, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res 2005. 65 (1) 177.85
- Matoba S, Kang JG, Patino WD, et al. P53 regulates mitochondrial respiration. Science. 2006.312 (5780) 1650-3.
- Ma W, Sung HJ. Park S Y, Matoba S, Hwang PM. A pivotal role for P53. Balancing aerobic respiration and glycolysis. J.Bioenergy Biomenhr. 2007. 39 (3) 243-6.
- J.W Shay, S Bacchetti. A survey of telomerase activity in human cancer. European Journal of Cancer, Vol 33. issue 5. April 1997. pages 789-791.
- Aubert G Landsorp PM. Telomeres and Aging. Physiological reviews. 2008.88. 557-579.
- Kim NW, Piatyzek MA, Prowse Kr, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrick SL, Shay JW. Specific association of human telomerase with immortal cells and cancer. Science. 1994. 266-2011-5.
- Soria J C, Gauthier LR, Raymond E, Granotier C, Morat L, Armand JP, Boussin FD, Sabatier L, Molecular detection of telomerase-positive circulating epithelial cells in metastatic breast cancer patients. Clin. Cancer Res. 1999. 917-5.
- Sapi E, Okpokwasili NI, Rutherford T. Detection of telomerase positive circulating epithelial cells in ovarian cancer patients. Cancer Detect Prev. 2002. 26.158-67.
- Goldkorn A, Ely B, Tangen CM, Tai YC. Xu T, Li, H, Twardowski P, Veldhuizen PJ, Aggarwal N, Carducci M, et al. Circulating tumor cell telomerase activity as a prognostic marker for overall survival in SWOG0421. A phase III metastatic castration-resistant prostate cancer trial. Intl. Journal of Cancer. 2015. 136- 1856-62.
- Mohammed A, Jafri Shakeel A, Ansari, and Jerry W. Shay. Roles of telomerase in cancer and advances in telomerase targeted therapies. Genome Med. 2016. 869.
- Paul Lieberman Ph.D. Brothers-in-Arms. How P53 and telomeres work together to stave off cancer. Science Daily (the Wistar Institute) January 15. 2016.
- R. Akeshuma. J. Kigawa, M Takahashi, T, Oishi, Y, Kasmainari, H, Itamochi, M, Shimado, S, Kamazawa, S, Sato, and N, Trerakwa. Telomerase activity and P53-dependent apoptosis in ovarian cancer cells. B.J.C. (2001) 84 (11) 1551-1555.
- Hoas A, Hepp HH, Kaul S. Albert T, Bastert G, Wallweiner D. Telomerase activity correlates with tumor aggressiveness and reflex therapy effect in breast cancer. Int. Journal Cancer 1998. 74-8-12.
- Brittney-Shea, Herbert Woodring, E Wright, and Jerry W Show. Telomerase and Breast Cancer. Breast Cancer Research 2001.3-146-149.
- Katarzyna Dobja-Kubica-Marzena Zalewska-Ziob Krysztof Brulinski, Agata Gawrychowska and Jacek Gawrychowska. Telomerase avtivity in non-small-cell-lung cancer. Polish Journal of Thoracic and Cardiovascular Surgery. 2016. 13. (1) 15-20.
- Hermann PC. Hubert SL, Herrler T, Aicher A, Ellwart JW, Cuba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007,1.313-23.
- S Colak and J.P. Medema. Cancer stem cells- important players in tumor therapy resistance. FEBS Journal, vol 281.no21, pp 4779-47791. 2014.
- Pedro M. Ponte and Andres Caicedo. Stemness in cancer. Stem Cells, Cancer Stem Cells, and their Microenvironment. Stem Cells International Volume 2017, Article ID5619472, 17 pages.
- Del Bufalo D, Rizzo A, Triscinoglio D. Cardinalu G, Torrisi MR, Zangemeister, Wittke U, Zupi G, Biroccio A. Involvement of hTERT in apoptosis induced by interference with BcL2 expression in function. Cell Death Differ. 2005.12.14. 29-38.
- Bernuidez Y, Erasso D, Johson N, Mattee AD. Lowell N, Kruk P. Telomerase confers resistance to caspase-mediated apoptosis. Clin Intervent Aging 2006. 1/15567.
- Serge Jurasunas N.D. MD> (Hom) and Olga Gakina Taylor Ph.D. How to Target Mutant P53 in a case of Multiple Cancer Recurrence. Townsend Letter August/Sept.2010. 68-71.
- Cosan DT, Soyocak A. Inhibiting telomerase activity and inducing apoptosis in cancer cells by several natural food compounds. Ir- Bibo L, editor. Review on selected topics of Telomere biology. InTech. Rijeka, Croatia. 2010. 123-148.
- Elisa Keating and Fatima Martel. Antimetabolic effects of polyphenols in breast cancer cells. Focus on glucose uptake and metabolism. Frontiers in Nutrition. 16 April. 2018.
- Natalia G, Vallianou, Angelos Evangelopoulous, Nikas Schizas, and Christos Kazazis. Potential anticancer Properties and Mechanisms of action of curcumin. Anticancer Research. February 2015. Vol35. No.2. 645-651
- Hanna Halysz, Natalia Lipinska, Blatej Rubis. Curcumin inhibits telomerase activity through human telomerase reverse transcriptase (TERT) in MCF-7 breast cancer cell line. Cancer Letter 184 (1) 1-6- 2012.
- Chakrabaity S, Ghosh U, Bhattacharyya NP, Bhattacharyya RK, Roy M. Inhibition of telomerase activity and induction of apoptosis by curcumin in K-526 cells. Molecular Mechanisms of Mutagenesis. 2006-596-81-90.
- Shankar Jagodeesh, Sataru Kyo, Partha P, Banerjee. Genistein represses Telomerase activity via both transcriptional and posttranscriptional Mechanism in the human cancer prostate. Cell Tumor and Stem Cell Biology. Feb.2006.
- Yanyan Li et all. Targeting cancer stem cells by curcumin and clinical applications. Cancer Letter. 346 (2) January 2014.
- Weifeng Huang, Chunpeng Wan, and Qi Luo. Genistein inhibits cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Intl Journal Mol Sci.2104 Mars 15 (3) 3432-3443.
- Serge Jurasunas. NK cell-based immunotherapy in the treatment of cancer using a new Arabinoxylan compound. Townsend Letter August/Sep 2019. 33-41.
- Nariman k. Badr, Said K. Areida, Kvan O, Ahmed, and Mamdooh Ghoneum. Arabinoxylan Rice Bran (MGn-3/Biobran enhances radiotherapy in animals bearing Ehrlich ascites carcinoma. Journal of Radiation Research. 2019. 1-12.
- Lanzilli G, Figetta M.P, Tricano M, et al. Resveratrol down regulates the growth of telomerase activity of breast cancer cells in vitro. Int. Journal. Oncol. 28.641-8. 2006 Mar.
- Sadova D, Whitlock E, Kane SE. The green tea polyphenol epigallocatechin-3-gallate inhibits telomerase and induce apoptosis in drug-resistant lung cancer. Biochemical and Biophysical Communication, 2007. 360-233-237.
- Sun L, Wang X. Effects of Allicin on both telomerase activity and apoptosis in gastric cancer. SGC. 7901. Cells. World J Gastroenterol. 20030-9- 1930-4.
- Moon D, Kand S, Kim K, Kim M. Choi YH, Kim G. Sulphoraphane decrease viability and telomerase activity in hepatocellular carcinoma. Hep 3B cells through the reactive oxygen species-dependent pathways. Cancer Letter 20110. 295 260-66.
- Erkan Yurtcu et al. Effects of silymarin and silymarin Doxorubicine Applications on Telomerase Activity of Human Hepatocellular Carcinoma Cell Line Hep G2. J Buon May 2015 20 (2) 555-561 (Journal of BUON in the official journal of the Balkan Univ of Oncology).
- Naasani I Oh, Hashi f, Oh, Hara T, Feng W Y, Johnston J, Chan K, Tsuruo T. Blocking Telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res 2003. 63-824-830
- Janet M. Pavese. Rebecca L. Farmer, Raymond C. Bergan. Inhibition of cancer cell invasion and metastasis by genistein. Cancer `Metastasis, Rev (2010n) 29.465-482
About the Author
The author can offer on request the two posters visible in the photo to doctors of Integrative Oncology.
Serge Jurasunas is an internationally well-known practitioner and researcher in complementary Oncology, nutrition, and molecular medicine with 53 years of experience practicing in a private clinic.
is considered as a pioneer in Naturopathic medicine, perfect some science such as Live Blood Analysis, oxidative dried blood test, and Iridology. He uses both blood tests for over 45 years and has spread its knowledge all over the world, delivered lectures in over 45 countries, and received several Academic Awards for his contribution to medical science and is a member of many professional associations in the US and Europe, such as the American Naturopathic Medical Association, the A4M, the New York Academy of Science. Dr. Jurasunas also received the Silver Medal for Research and Invention by the French Academy. He was a former professor of Integrative Medicine at Capital University of Integrative in Washington, D.C., and currently is Professor of Naturopathic Oncology at Pan American University of Sciences and Natural Medicine.
During the past 12 years, he has concentrated on the research and clinical application of molecular markers especially the P53 tumor suppressor gene now associate with glycolysis, immune defense, and telomerase activity. He developed an important library on the P53 gene associated with cancer and telomerase for references. He was invited twice in the Republic of China to present a paper on P53 tumor suppressor associate with cancer include at the 2nd International Congress on Complementary Oncology in Munich organized by the German Society of Oncology. Lately, he started to include telomerase in the testing of cancer patients to determine the exact ratio with the P53 gene which permits to make a better diagnosis of the patient, prevent eventually from metastasis, and follow treatment using selective dietary compounds. This is a new and scientific way to diagnose cancer and to prove the value of the applied therapy.
He has been a frequent contributor to the Townsend Letter contributor for over 21 years and authored a major book in English (3 in French and 4 in Portuguese), “Health and Disease Begin in the Colon” (Amazon) and a new one coming up in the U.S.: “ Cancer Treatment Breakthrough – Immuno Oncology using Rice Bran Arabinoxylan.”
How to contact Professor Dr. Serge Jurasunas
Throgh contact form by click here